The independent Site Management Organisations (SMOs) which run clinical trials on behalf of the pharmaceutical industry continue to expand not only the number of trials being brought to the UK but also the number of patients enrolled.
While The Association of the British Pharmaceutical Industry (ABPI) reported that there was a substantial decline in patient numbers at NHS sites (NIHR CRN 1) independent SMOs are expanding both their networks and patients enrolled.
Chris Dodd, Chief Commercial Officer of Panthera Biopartners – a leader in this field, commented “The SMOs which recruit tens of thousands of patients each year to clinical trial sites across the UK not only continued to run trials during the pandemic – including many of the major vaccine studies – but also rapidly recovered from COVID and are providing global pharmaceutical companies with access to more and more UK patients.
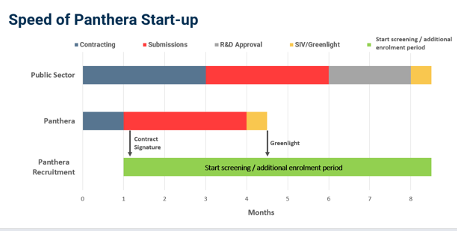
However, the UK NHS sites have not only been closed to clinical trials for years but are also very slow in moving from discussion to enrolment. At Panthera we can start recruitment seven months before an NHS site. This delay can cost a pharma billions of lost revenue as the patent runs out and they lose the last seven months of product sales.”
The UK is considered to be one of the most desirable places to undertake clinical trials due to the depth of medical expertise and the diversity and density of the population. This is not only of great benefit to patients who get access to the latest medicines but also to the UK life science industry which remains one of the world leaders.
1 NIHR CRN National Institute for Health and Care Research Clinical Research Network
