Panthera’s SMO model delivers market-leading patient recruitment
for vaccine trials
In the post-COVID-19 period, the UK has emerged as a world leader in vaccine trials, but data from two case studies shows that Panthera is not only outperforming global sites, but also competitor sites across the UK.
In a recent COVID-19 booster healthy volunteer vaccine trial, Panthera’s Rochdale, Preston, and Sheffield sites were ranked 1st, 2nd and 4th top recruiting sites in the UK. Despite only representing 2% of the global trial sites, Panthera sites were also responsible for 6% of all patient randomisations. On average, Panthera delivered 2.9 times more randomisations than competing UK sites and 2.8 times more randomisations than competing global sites.
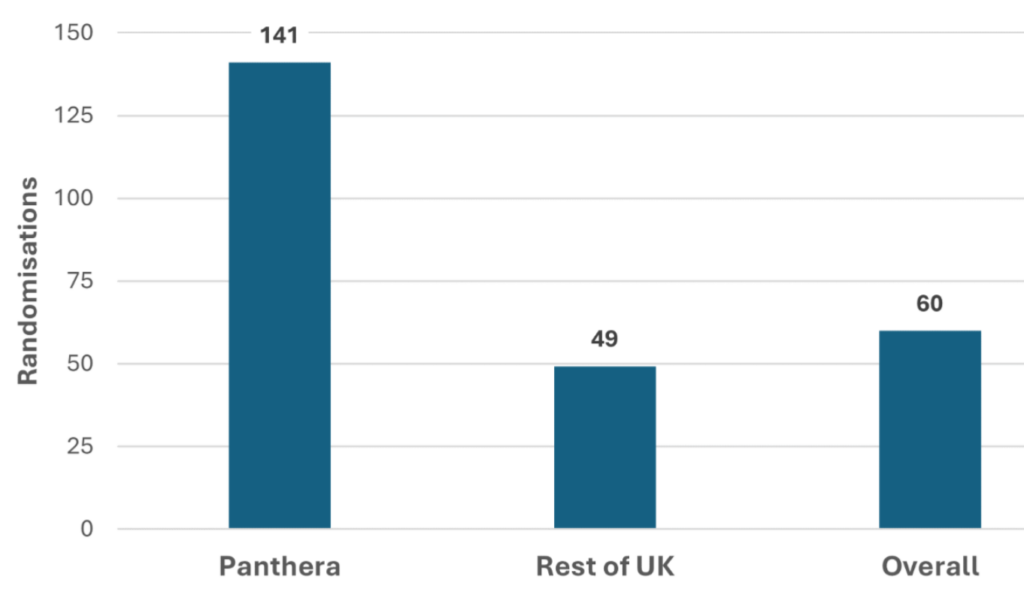
Similarly, in an Influenza healthy volunteer vaccine trial, Panthera sites were responsible for 60% of the total trial sites and 95% of total randomisations for the trial.
These results are why Panthera has rapidly established such a strong reputation for vaccine trial recruitment, and demonstrates the key benefits of the SMO model when it comes to recruiting healthy volunteers. With the global health community on high alert for the next pandemic, the capacity to rapidly and effectively recruit for vaccine trials is going to remain a high priority for the future.
COMING SOON: Panthera’s new Oncology clinical trials solution!
Panthera is delighted to announce that it is re-entering the Oncology clinical trials space.
Further details will be revealed soon but if you’re interested, and want to get ahead of the game and learn more, then please reach out to our team by emailing [email protected]
Feel free to ask if you have any questions or would like further information.
Stay Informed – To receive updates directly to your inbox, register HERE
Together, let’s improve patient outcomes and advance cancer research
PANTHERA IN THE NEWS
International Clinical Trials: Are decentralised clinical trials a myth of the medical world?
Decentralised clinical trials are gaining popularity, but not all are convinced they work – or even exist.
Panthera’s Ian Smith and Chris Dodd explore this topic in the Spring issue of International Clinical Trials (ICT). We thought you may be interested to read the article.
The article can be found HERE
Credit: International Clinical Trials, May 2024, pages 20-22. © Samedan Ltd
Panthera dinners – watch this space!
Our last dinner was scheduled to take place on the 18th June with guest speaker Andew Griffith – Minister of State for the Department for Science, Innovation and Technology. However, due to the General Election and the rules of purdah, we were unfortunately not able to proceed.
Please watch this space for details of our next dinner which will take place later this year.
